INFLAMMATORY BOWEL DISEASE
 Inflammatory bowel disease (IBD) is a spectrum of chronic idiopathic inflammatory intestinal conditions. IBD causes significant gastrointestinal symptoms that include diarrhoea, abdominal pain, bleeding, anaemia, and weight loss. IBD also is associated with a spectrum of extra intestinal manifestations, including arthritis, ankylosing spondylitis, sclerosing cholangitis, uveitis, iritis, pyoderma gangrenosum, and erythema nodosum.
Inflammatory bowel disease (IBD) is a spectrum of chronic idiopathic inflammatory intestinal conditions. IBD causes significant gastrointestinal symptoms that include diarrhoea, abdominal pain, bleeding, anaemia, and weight loss. IBD also is associated with a spectrum of extra intestinal manifestations, including arthritis, ankylosing spondylitis, sclerosing cholangitis, uveitis, iritis, pyoderma gangrenosum, and erythema nodosum.
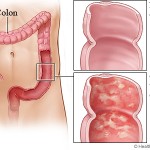
IBD conventionally is divided into two major subtypes: ulcerative colitis and Crohn’s disease. Ulcerative colitis is characterized by confluent mucosal inflammation of the colon starting at the anal verge and extending proximally for a variable extent (e.g., proctitis, left-sided colitis, or pancolitis). Crohn’s disease, by contrast, is characterized by transmural inflammation of any part of the gastrointestinal tract but most commonly the area adjacent to the ileocecal valve. The inflammation in Crohn’s disease is not necessarily confluent, frequently leaving “skip areas” of relatively normal mucosa. The transmural nature of the inflammation may lead to fibrosis and strictures or, alternatively, fistula formation.
Medical therapy for IBD is problematic. Because no unique abnormality has been identified, current therapy for IBD seeks to dampen the generalized inflammatory response; however, no agent can reliably accomplish this, and the response of an individual patient to a given medicine may be limited and unpredictable. Based on this variable response, clinical trials generally employ standardized quantitative assessments of efficacy that take into account both clinical and laboratory parameters (e.g., the Crohn’s Disease Activity Index). The disease also exhibits marked fluctuations in activity¾even in the absence of therapy¾leading to a significant “placebo effect” in therapeutic trials.
Specific goals of pharmacotherapy in IBD include controlling acute exacerbations of the disease, maintaining remission, and treating specific complications such as fistulas. Specific drugs may be better suited for one or the other of these aims. For example, steroids remain the treatment of choice for moderate to severe flares but are inappropriate for long-term use because of side effects and their inability to maintain remission. Other immunosuppressives, such as azathioprine, that require several weeks to achieve their therapeutic effect have a limited role in the acute setting but are preferred for long-term management.
For many years glucocorticoids and sulfasalazine were the mainstays of medical therapy for IBD. More recently, medicines used in other immune/inflammatory conditions, such as azathioprine and cyclosporine, have been adapted for IBD therapy. A more thorough appreciation of the intricacies of the inflammatory response and improved biotechnology have led to the development of biological agents that can target single steps in the immune cascade. Drug delivery to the appropriate site(s) along the gastrointestinal tract also has been a major challenge, and second-generation agents have been created with improved drug delivery, increased efficacy, and decreased side effects.
Crohn’s disease and ulcerative colitis are chronic idiopathic inflammatory disorders of the GI tract; a summary of proposed pathogenic events and potential sites of therapeutic intervention. While Crohn’s disease and ulcerative colitis share a number of gastrointestinal and extra intestinal manifestations and can respond to a similar array of drugs, emerging evidence suggests that they result from fundamentally distinct pathogenetic mechanisms. Histologically, the transmural lesions in Crohn’s disease exhibit marked infiltration of lymphocytes and macrophages, granuloma formation, and submucosal fibrosis, whereas the superficial lesions in ulcerative colitis have lymphocytic and neutrophilic infiltrates. Within the diseased bowel in Crohn’s disease, the cytokine profile includes increased levels of interleukin-12 (IL-12), interferon-g, and tumours necrosis factor-a (TNF-a), findings characteristic of T-helper 1 (TH1)-mediated inflammatory processes. In contrast, the inflammatory response in ulcerative colitis resembles more closely that mediated by the TH2 pathway.
Important insights into pathogenesis also have emerged from genetic analyses of Crohn’s disease. Mutations in a gene called nucleotide-binding oligomerization domain-2 (NOD2, also called CARD15) are associated with both familial and sporadic Crohn’s disease in Caucasians. NOD2 is expressed in monocytes, granulocytes, dendritic cells, and epithelial cells. It is proposed to function as an intracellular sensor for bacterial infection by recognizing peptidoglycans, thereby playing an important role in the natural immunity to bacterial pathogens. Consistent with this model, other studies have identified bacterial antigens, including pseudomonal protein I2 and a flagellin protein, as dominant super antigens that induce the TH1 response in Crohn’s disease (shown as bacterial products. Thus these converging experimental approaches are generating novel insights into the pathogenesis of Crohn’s disease that soon may translate into novel therapeutic approaches to IBD.
First-line therapy for mild to moderate ulcerative colitis generally involves mesalamine (5-aminosalicylic acid, or 5-ASA). The archetype for this class of medications is sulfasalazine, which consists of 5-ASA linked to sulfapyridine by an azo bond. Although this drug was developed originally as therapy for rheumatoid arthritis, clinical trials demonstrated a beneficial effect on the gastrointestinal symptoms of subjects with concomitant ulcerative colitis. Sulfasalazine represents one of the first examples of an oral drug that is delivered effectively to the distal gastrointestinal tract. Given individually, either 5-ASA or sulfapyridine is absorbed in the upper gastrointestinal tract; the azo linkage in sulfasalazine prevents absorption in the stomach and small intestine, and the individual components are not liberated for absorption until colonic bacteria cleave the bond. 5-ASA is now regarded as the therapeutic moiety, with little, if any, contribution by sulfapyridine.
The effects of glucocorticoids on the inflammatory response are numerous and well documented. Although glucocorticoids are universally recognized as effective in acute exacerbations, their use in either ulcerative colitis or Crohn’s disease involves considerable challenges and pitfalls, and they are indicated only for moderate to severe IBD. Because the same issues have an impact on steroid use in both ulcerative colitis and Crohn’s disease, they are addressed together.
Several drugs developed initially for cancer chemotherapy or as immunosuppressive agents in organ transplants have been adapted for treatment of IBD. While their initial use in IBD was based on their immunosuppressive effects, their specific mechanisms of action are unknown. Increasing clinical experience has defined specific roles for each of these medicines as mainstays in the pharmacotherapy of IBD. However, both real and potential side effects mandate a careful assessment of potential risks and benefits in each patient.
By: Ammarah Khan




Currently it sounds like Movable Type is the top blogging platform available right now. (from what I’ve read) Is that what you’re using on your blog?
No, we have our own.
Thank You