ALZHEIMER’S DISEASE (AD)
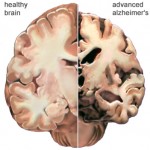 AD produces an impairment of cognitive abilities that is gradual in onset but relentless in progression. Impairment of short-term memory usually is the first clinical feature, whereas retrieval of distant memories is preserved relatively well into the course of the disease. As the condition progresses, additional cognitive abilities are impaired, among them the ability to calculate, exercise visuospatial skills, and use common objects and tools (ideomotor apraxia).
AD produces an impairment of cognitive abilities that is gradual in onset but relentless in progression. Impairment of short-term memory usually is the first clinical feature, whereas retrieval of distant memories is preserved relatively well into the course of the disease. As the condition progresses, additional cognitive abilities are impaired, among them the ability to calculate, exercise visuospatial skills, and use common objects and tools (ideomotor apraxia).
The level of arousal or alertness of the patient is not affected until the condition is very advanced, nor is there motor weakness, although muscular contractures are an almost universal feature of advanced stages of the disease. Death, most often from a complication of immobility such as pneumonia or pulmonary embolism, usually ensues within 6 to 12 years of onset. The diagnosis of AD is based on careful clinical assessment of the patient and appropriate laboratory tests to exclude other disorders that may mimic AD; at present, no direct ante mortem confirmatory test exists.
AD is characterized by marked atrophy of the cerebral cortex and loss of cortical and subcortical neurons. The pathological hallmarks of AD are senile plaques, which are spherical accumulations of the protein b-amyloid accompanied by degenerating neuronal processes, and neurofibrillary tangles, composed of paired helical filaments and other proteins Although small numbers of senile plaques and neurofibrillary tangles can be observed in intellectually normal individuals, they are far more abundant in patients with AD, and the abundance of tangles is roughly proportional to the severity of cognitive impairment.
In advanced AD, senile plaques and neurofibrillary tangles are numerous and most abundant in the hippocampus and associative regions of the cortex, whereas areas such as the visual and motor cortices are relatively spared. This corresponds to the clinical features of marked impairment of memory and abstract reasoning, with preservation of vision and movement. The factors underlying the selective vulnerability of particular cortical neurons to the pathological effects of AD are not known.
The neurochemical disturbances that arise in AD have been studied intensively. Direct analysis of neurotransmitter content in the cerebral cortex shows a reduction of many transmitter substances that parallels neuronal loss; there is a striking and disproportionate deficiency of acetylcholine. The anatomical basis of the cholinergic deficit is the atrophy and degeneration of subcortical cholinergic neurons, particularly those in the basal forebrain (nucleus basalis of Meynert), that provide cholinergic innervation to the whole cerebral cortex.
The selective deficiency of acetylcholine in AD, as well as the observation that central cholinergic antagonists such as atropine can induce a state that bears some resemblance to the dementia of AD, has given rise to the “cholinergic hypothesis,” which proposes that a deficiency of acetylcholine is critical in the genesis of the symptoms of AD. Although the conceptualization of AD as a “cholinergic deficiency syndrome” in parallel with the “dopaminergic deficiency syndrome” of PD provides a useful framework, it is important to note that the deficit in AD is far more complex, involving multiple neurotransmitter systems, including serotonin, glutamate, and neuropeptides, and that in AD there is destruction of not only cholinergic neurons but also the cortical and hippocampal targets that receive cholinergic input.
The presence of aggregates of b-amyloid is a constant feature of AD. Until recently, it was not clear whether the amyloid protein was causally linked to the disease process or merely a by-product of neuronal death. The application of molecular genetics has shed some light on this question. B-amyloid from affected brains and found to be a short polypeptide of 42 to 43 amino acids. This information led to cloning of amyloid precursor protein (APP), a much larger protein of more than 700 amino acids, which is expressed widely by neurons throughout the brain in normal individuals as well as in those with AD.
The function of APP is unknown, although the structural features of the protein suggest that it may serve as a cell surface receptor for an as-yet-unidentified ligand. The production of b-amyloid from APP appears to result from abnormal proteolytic cleavage of APP by the b-site APP-cleaving enzyme BACE. This may be an important target of future therapies.
Analysis of APP gene structure in pedigrees exhibiting autosomal dominant inheritance of AD has shown that in some families, mutations of the b-amyloid-forming region of APP are present, whereas in others, mutations of proteins involved in the processing of APP are implicated. These results suggest that it is possible for abnormalities in APP or its processing to cause AD.
The vast majority of cases of AD, however, are not familiar, and structural abnormality of APP or related proteins has not been observed consistently in these sporadic cases of AD. As noted earlier, common alleles of the Apo E protein have been found to influence the probability of developing AD. Many investigators believe that modifying the metabolism of APP might alter the course of AD in both familial and sporadic cases, but no clinically practical strategies have been developed.
A major approach to the treatment of AD has involved attempts to augment the cholinergic function of the brain. An early approach was the use of precursors of acetylcholine synthesis, such as choline chloride and phosphatidyl choline (lecithin). Although these supplements generally are well tolerated yet randomized trials have failed to demonstrate any clinically significant efficacy.
A somewhat more successful strategy has been the use of inhibitors of acetyl cholinesterase (AChE), the catabolic enzyme for acetylcholine. Physostigmine, a rapidly acting, reversible AChE inhibitor, produces improved responses in animal models of learning, and some studies have demonstrated mild transitory improvement in memory following physostigmine treatment in patients with AD. The use of physostigmine has been limited because of its short half-life and tendency to produce symptoms of systemic cholinergic excess at therapeutic doses.
Four inhibitors of AChE currently are approved by the FDA for treatment of Alzheimer’s disease: tacrine, donepezil , rivastigmine , and galantamine. Tacrine is a potent centrally acting inhibitor of AChE. Studies of oral tacrine in combination with lecithin have confirmed that there is indeed an effect of tacrine on some measures of memory performance, but the magnitude of improvement observed with the combination of lecithin and tacrine is modest at best . The side effects of tacrine often are significant and dose-limiting; abdominal cramping, anorexia, nausea, vomiting, and diarrhoea are observed in up to one-third of patients receiving therapeutic doses, and elevations of serum transaminases are observed in up to 50% of those treated. Because of significant side effects, tacrine is not used widely clinically.
Donepezil is a selective inhibitor of AChE in the CNS with little effect on AChE in peripheral tissues. It produces modest improvements in cognitive scores in Alzheimer’s disease patients and has a long half-life, allowing once-daily dosing. Rivastigmine and galantamine are dosed twice daily and produce a similar degree of cognitive improvement. Adverse effects associated with donepezil, rivastigmine, and galantamine are similar in character but generally less frequent and less severe than those observed with tacrine; they include nausea, diarrhoea, vomiting, and insomnia. Donepezil, rivastigmine, and galantamine are not associated with the hepatotoxicity that limits the use of tacrine.
An alternative strategy for the treatment of AD is the use of the NMDA glutamate-receptor antagonist memantine (NAMENDA). Memantine produces a use-dependent blockade of NMDA receptors. In patients with moderate to severe AD, use of memantine is associated with a reduced rate of clinical deterioration. Whether this is due to a true disease-modifying effect, possibly reduced excitotoxicity, or is a symptomatic effect of the drug is unclear. Adverse effects of memantine usually are mild and reversible and may include headache or dizziness.
By: Ammarah Khan



